What is ReSPoND?
Each year about 800,000 babies in the UK have a blood test taken to screen for specific conditions, which if treated early will improve the child’s health and well-being (known as newborn screening (NBS)). In 2018-19, over 10,000 babies were identified as being affected or healthy carriers of a gene for one of the conditions screened for, which include sickle cell disease, cystic fibrosis, metabolic diseases and hypothyroidism.
Parents’ agree to the blood test at the time of birth but many never expect a positive result. When a positive result occurs, a variety of ways are used to deliver the result but many parents complain about the manner in which the information is communicated to them. With the expansion of NBS in England, the importance of delivering this information appropriately to minimise any long-term negative health and psychological consequences is vital.
Our Aim: For parents and health professionals to work together to design interventions to facilitate effective communication of positive NBS results to parents by health professionals.
We are working with parent representatives for each screened condition covered by NBS who will form an advisory group to assist the research team, provide feedback on each phase of the project and monitor its progress. Charities whose work focuses on the conditions screened for have provided their support for this work.
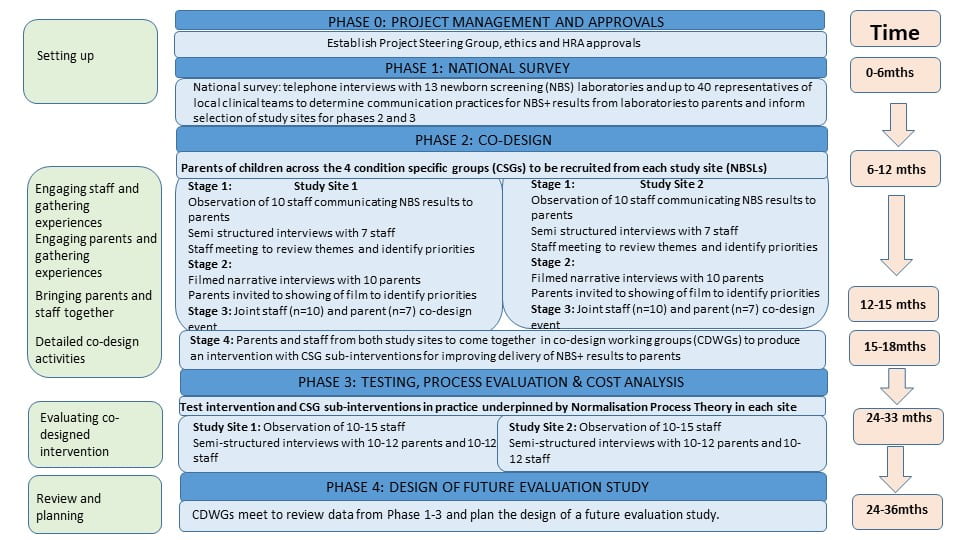
The research takes place in four phases:
Phase 0: Gaining the necessary approvals to do the work
Phase 1: What is happening now?
We will find out how NBS results are communicated from laboratories to parents in all parts of England for the screened conditions. This will help us understand differences in terms of current practices and associated benefits. It will also help us decide which sites will be used for phases 2 and 3.
Phase 2: Parents and health professionals working together to design the best way(s) to communicate positive NBS results
We will watch how NBS results are delivered to parents and then ask parents and health professionals about their experiences in two study sites. We will then develop a composite video of parental experiences. Following this we will ask parents and health professionals to work together through a series of workshops to design approaches (interventions) for the effective and sensitive communication of positive NBS results to parents of affected children.
Phase 3: Evaluating the different approaches
We will start using the new approaches designed in Phase 2 in the two study sites and compare them to usual practice observed in Phase 2. We will watch how the new approaches are used and ask parents and health professionals which approach they think is best, how it affects them and work out how much each will cost, who needs to be involved and any factors that may affect how well the approaches work in practice such as language barriers. We will compare costs associated with the new, co-designed interventions with existing practices described in Phase 1.
Phase 4: Future Directions
Information from the first three phases will be reviewed and plans made for a future study to evaluate the chosen approach.
Dissemination: The findings will be shared with support groups and charities, along with health professionals and academics.
This study is funded by the National Institute for Health Research (NIHR) HS&DR (project reference 16/52/25). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.